We are developing a pipeline of next-generation precision therapies designed to meet significant needs of people with cancer.
Taletrectinib – Phase 2
a next-generation ROS1 inhibitor
ROS1-positive non-small cell lung cancer (NSCLC)
Safusidenib – Phase 2
a mIDH1 inhibitor
IDH1-mutant Grades 2 and 3 glioma, cholangiocarcinoma and other tumors
We are also advancing a number of preclinical precision oncology programs.
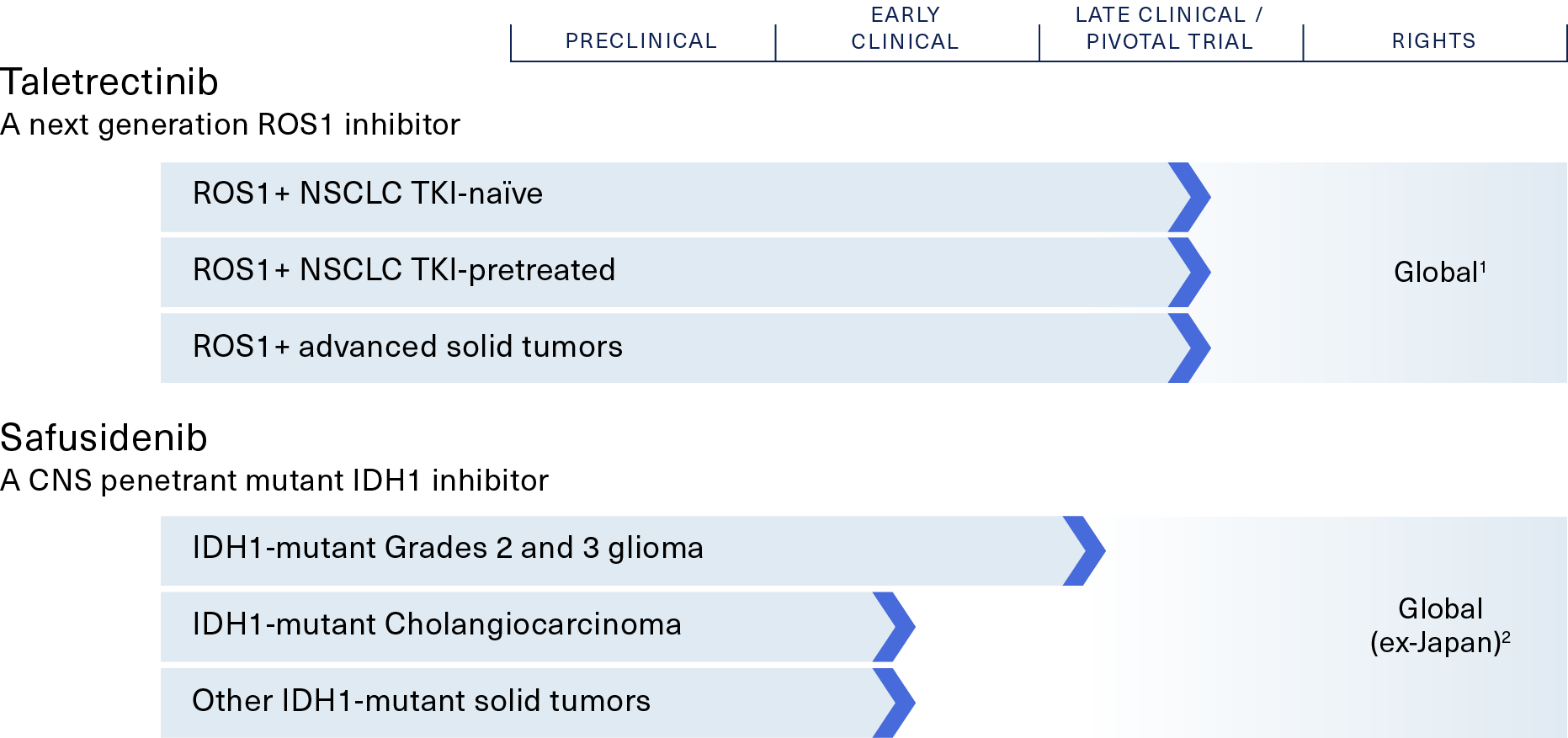
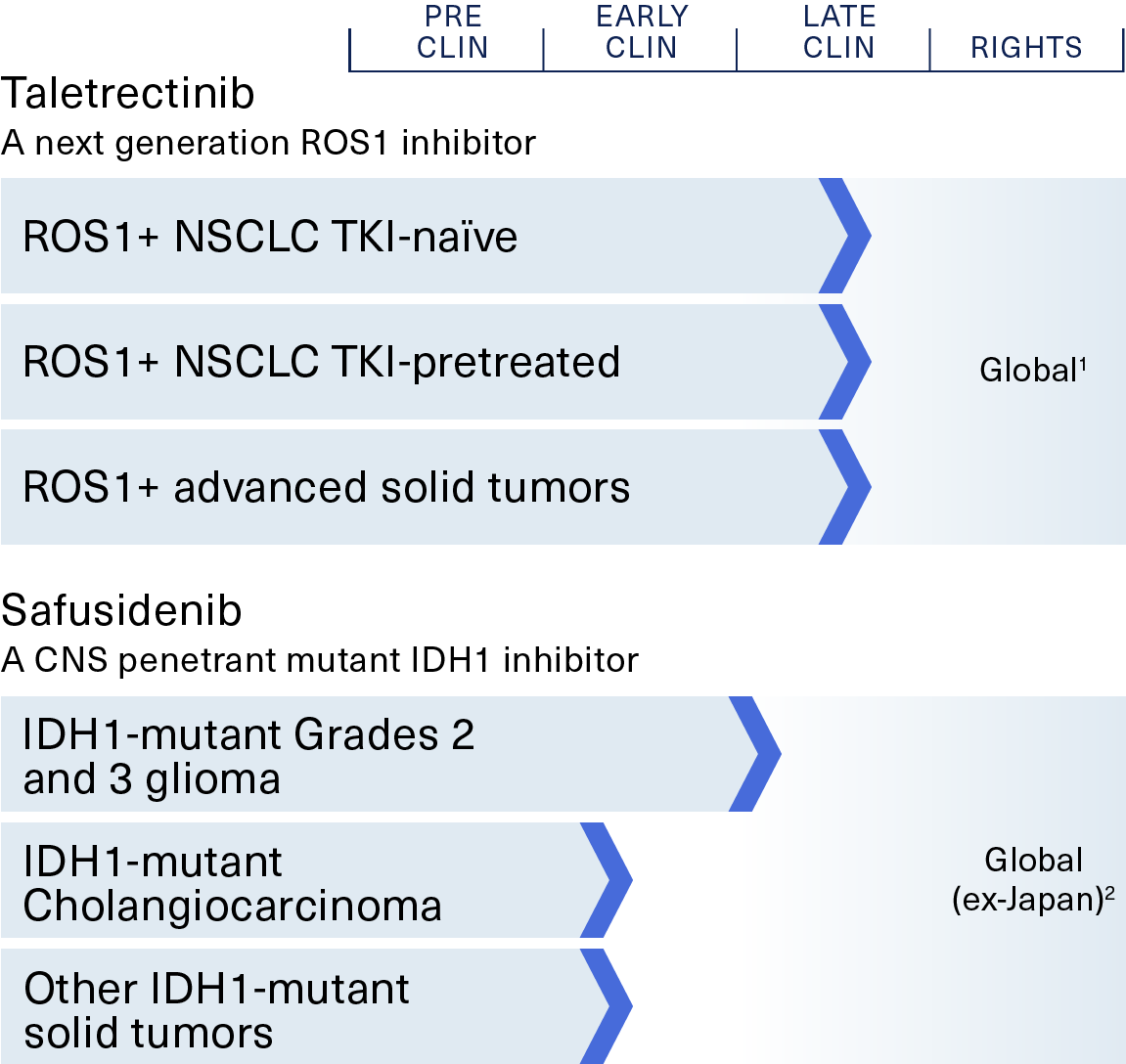
1. AnHeart owns global rights to taletrectinib; AnHeart licensed commercial rights to Innovent, Nippon Kayaku, and NewG Lab, in Greater China, Japan, and Korea, respectively
2. AnHeart owns global rights to safusidenib excluding Japan. Daiichi Sankyo retains development and commercial rights in Japan
TALETRECTINIB (ROS1 INHIBITOR)
Taletrectinib is an oral, potent, brain penetrant, selective, next-generation potential best-in-class ROS1 inhibitor being evaluated for the treatment of ROS1-positive NSCLC in people who have not previously received treatment with a tyrosine kinase inhibitor (TKI-naïve), as well as people who have previously received treatment with a TKI.
Taletrectinib has been granted Breakthrough Therapy Designation by both the U.S. Food and Drug Administration (FDA) and the China National Medical Products Administration (NMPA) for the treatment of patients with advanced or metastatic ROS1-positive NSCLC.
Safusidenib (mIDH1 inhibitor)
Safusidenib is an investigational, brain-penetrant, mIDH1 inhibitor being evaluated for multiple solid tumors with IDH1 mutations.

